Genetic Abnormalities can occur when there are problems in the genes or DNA of a growing fetus. This could lead to genetic disorders such as Down Syndrome, Patau Syndrome, Edward Syndrome, etc. Down Syndrome is the most common genetic disorders among newborns, with an incidence rate of 1/660 live births in Malaysia1.
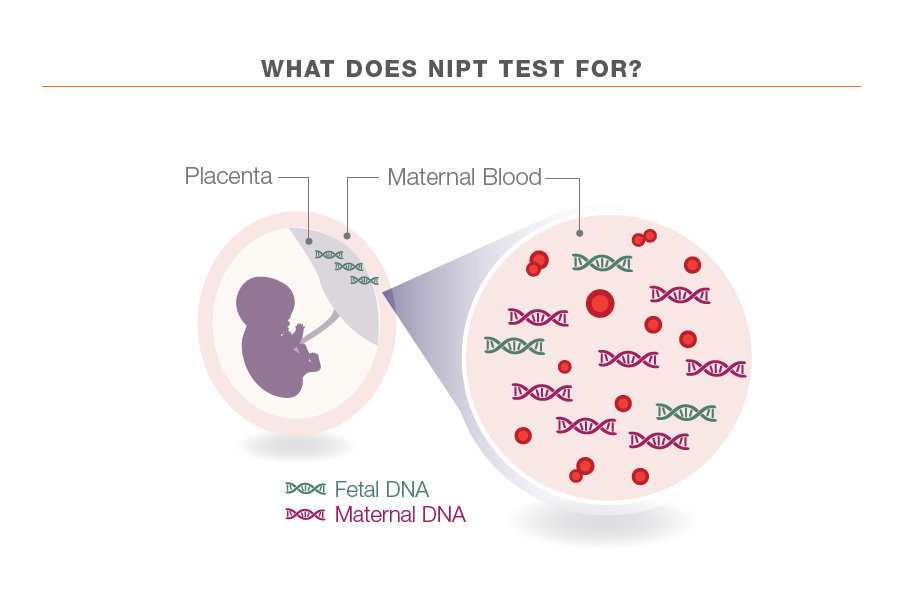
Non-invasive Prenatal Test (NiPT) is an emerging test that screens for some of the most common genetic abnormalities that can affect the health and development of a baby. This is done via a blood sample of the pregnant mother as it contains DNA from both mother and baby. Cells from the placenta (cell-free fetal DNA), which is usually the same as the baby, gets shed into the mother’s blood stream during pregnancy and these cell-free fetal DNA is extracted from the blood collected for further analysis.
NiPT offers many advantages over conventional screening and testing, including, but not limited to:
- NiPT detects conditions that are not tested in other conventional tests.
- NiPT screens for Down Syndrome with an accuracy rate greater than 99%, compared to conventional screening that provides only up to 95%2.
- NiPT poses no risk to the fetus as blood is drawn from the mother unlike procedures like Amniocentesis and Chorionic Villus Sampling (CVS), which have miscarriage risk of up to 1% and 2%, respectively3.
Pregnant mothers in high risk groups are commonly offered NiPT by their physicians. However, the American College of Obstetricians and Gynaecologists (ACOG) recommends that all pregnant women, regardless of risk should be offered all prenatal tests that are available4.
NiPT can be undertaken as early as 10 weeks of pregnancy and at any point thereafter before the baby is born. Doctors will receive the results within 10 - 14 days.
Currently, Quantum Diagnostics offers several types of NiPT as follow:
References:
