Order Of Draw
Blood culture tubes (applying full aseptic technique)
Blue cap (sodium citrate)
Yellow cap (Serum Separator Tube)
Green Cap (Heparinized tube)
Purple cap (EDTA tube)
Royal Blue cap

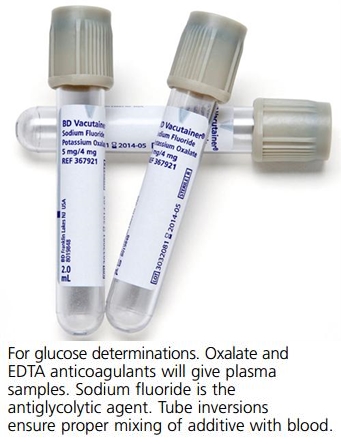
Grey cap (fluoride tube)
Unless indicated samples should be stored in room temperature (air conditioned) and away from sunlight until pick up.
Tubes must be labeled with patient’s full name.
Microbiology
| SPECIMEN TYPE | COLLECTION GUIDELINES | CONTAINER & STORAGE | COMMENTS |
|---|---|---|---|
| Abscess/Wound Open Closed |
Remove surface exudate by wiping with sterile saline Aspirate if possible. Place fluid or tissue in sterile container Syringes are acceptable if delivered promptly
Aspirate if possible. If swab used, obtain at the time of incision, drainage or debridement of wound. |
Sterile container/ swab transport medium/ sterile syringe/ Room Temperature | Tissue or fluid samples are preferable to swabs. Sampling of the surface area may contaminate the sample with flora not involved in the infection. |
| Biopsy/Bone/Tissue | Submit in sterile container without formalin Specimen may be kept moist with 0.85% sterile saline | Sterile container Room Temperature | Sampling of the surface area may contaminate with normal flora not involved in the infection. |
| Sterile Body Fluids Abdominal Ascites, Bile, Synovial, Pericardial, Peritoneal, Pleural |
|
Sterile container/blood culture bottle Room Temperature |
Fluid samples are preferable to swabs dipped in fluid |
| CSF | The physicians generally obtains these samples | Sterile container Room Temperature |
Nil |
| Blood Culture | ASEPTIC TECHNIQUE IS CRITICAL TO PROPER BLOOD CULTURE COLLECTION Clean venipuncture site using 70% alcohol then with 10% iodine. Draw blood from the cleaned site : 8-10ml blood for adults, 1-3 ml blood for children |
Blood culture bottle Room Temperature |
A blood culture set consists of an aerobic and anaerobic bottle for adults and older children. For children, a set is a single pediatric bottle. Blood volume must be 8-10ml for adults and 1-3ml for children. |
| Ear swab |
|
Sterile gel swab/VTM Room Temperature Refrigerate |
Nil If otitis externa is suspected, vigorous swabbing is needed as simple surface swabbing may miss a streptococcal infection. |
| Eye , conjunctiva |
|
Sterile gel swab Room Temperature |
Nil |
| Eye, Cornea (scrapings) |
|
Sterile gel swab Room Temperature |
Nil |
| Respiratory Tract, Upper Nasal Swab Oral |
Viral : viral transport media (VTM) |
Room Temperature | Nil |
| Throat |
Bacterial/Fungus : routine culture swab Viral : viral transport media (VTM) |
Refrigerate Sterile Gel Swab/VTM Room Temperature Refrigerate |
Nil |
| ERespiratory Tract, Lower Sputum, expectorated Tracheal aspirate |
|
Sterile gel swab/VTM Room Temperature Refrigerate |
Specimens must be brought to the laboratory as soon as possible after collection. |
| Skin Scrapping |
|
Sterile container Room Temperature |
Nil |
| Nail |
|
Sterile gel swab/VTM Room Temperature Refrigerate |
Nil |
| Gastrointestinal Tract |
|
Sterile container Room Temperature Refrigerate |
All stool specimens must be brought to laboratory as soon as possible. |
| Rectal swab | Bacterial/Fungus : routine culture swab Viral Culture: Viral Transport Media (VTM) available from HQ. |
Room Temperature Room Temperature Refrigerate |
Nil |
| Urine | Collect 20ml of mid-stream urine (MSU) in sterile specimen container. | Sterile urine container Refrigerate |
Nil |
Cytology
General Instructions on Specimen Fixation
-
Smears
Rapid fixation of smears is necessary to preserve cytological detail. If smears are allowed to dry on the slides prior to fixation, marked distortion of cells occurs.
Smear preparation such as vaginal, cervical, endometrial aspirations and aspiration cytology specimens should be fixed immediately in a solution of 95% ethyl alcohol or a coating fixative such as Cytofix; other commercial spray fixatives may be used. A minimum of fifteen to twenty minutes fixation is required in the case of ethyl alcohol fixation (although prolonged fixation will not materially alter the cytology). In the case of spray fixatives, the smear should be allowed to dry for 10 minutes prior to placing into slide holders for dispatch to the laboratory.
The slide MUST be labeled with the patient name and identification number (last 6 digits), passport or MRN.
Histology
A Request Form must accompany all specimens. When more than one specimen is sent from the same patient at the same operation, use only one form. Specimens from different anatomical sites should be sent in separate containers and must be itemized in the same Request Form. The containers of such samples must be similarly itemized and labeled with patient’s identification such that they can be cross-referenced to the patient and the anatomic site and side of origin of the sample.
Special Instructions
- Frozen sections will be done without fixative. Advance notice is required. Kindly contact the laboratory for specific appointment
- Routine specimens should be sent in containers with 10% buffered formalin unless otherwise stated.
- Appropriate containers are available from the laboratory on request. To prevent leakage it is advised to double wrap the specimen container.
- Multiple small specimens, such as gastrointestinal biopsies, should be mounted on a piece of filter paper and properly labeled.
- Large specimens such as colon must be completely immersed in formalin. Containers must be tightly secured.
- Do not crush specimens with forceps, hemostats or other instruments. Avoid using cautery.
- Do not force a large specimen into a small container. Large specimens must be completely surrounded by formalin for proper fixation.
- URGENT REPORT” request has to be clearly indicated on the request form; otherwise they will be processed as per according to queue.
- For specimens where orientation is important, mark or tag the specimen e.g. Axillary tail of mastectomy specimens, orientation of surgical margin.
